Iodoform Test for Alcohols
Iodoform test is used to check the presence of carbonyl compounds with the structure R-CO-CH 3 or alcohols with the structure R-CHOH-CH 3 in a given unknown. A positive result the pale yellow precipitate of triiodomethane iodoform is given by an alcohol containing the grouping.

Iodoform Test Description And Mechanism Compounds That Test Positive
Qualitative test for alcohol- there are various tests to detect the alcoholic group present-Ester test.
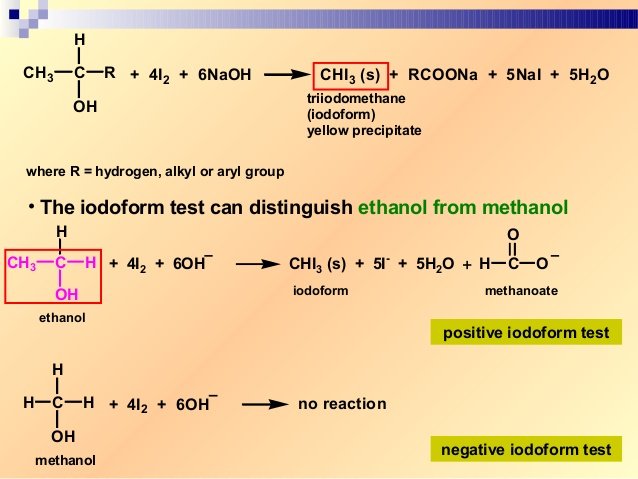
. The iodoform test is a test that is conducted successfully with secondary alcohols acetaldehyde and ketones. The given organic compound is heated in the presence of a sodium. The iodoform haloform test for alcohols with the structure CH3CHOH-R is both demonstrated and explained.
Secondary alcohols ketones and acetaldehyde show a positive iodoform test. The formation of white fumes indicates the presence of alcohol. Private tuition online from franklychemistry.
This test does not distinguish 1 2 3 alcohol but is specific for only one class of alcohol. What the Triiodomethane Iodoform Reaction Shows. The substrate you have provided 2-butanol would indeed give a positive test.
The test is given by all those compounds in which a methyl group is. First the compound is. A yellow precipitate of iodoform is formed when the given compound is heated.
Test For Alcohol Click Here for Sample Questions Along with Ethanol some secondary alcohols that consist of at least one methyl group in the alpha position test positive. There are two apparently quite different mixtures of reagents that can be. Iodoformtest is used to check the presence of carbonyl compounds with the structure R-CO-CH3 or alcohols with the structure R-CHOH-CH3 in a given unknown.
The iodoform test is performed in presence of. Ceric ammonium nitrate test. Iodoform Test The iodine test is an important test used to detect the presence of acyl group in a compound.
In the iodoform test sodium. The triiodomethane iodoform reaction can be used to identify the presence of a CH 3CHOH group in alcohols. Iodoform test is used to test for the presence of either methyl ketone group CH 3 CO or secondary alcohol CH 3 CHOH.
Iodoform Test This test is slightly different from the previous three tests. This test is given by secondary alcohols ketones and acetaldehyde. The iodoform test is used to determine whether a given unknown chemical contains carbonyl compounds such as aldehydes and ketones with the structure R-CO-CH3 or alcohols with the.
Which isomer of butyl alcohol respond to iodoform test.

Iodoform Test Description And Mechanism Compounds That Test Positive
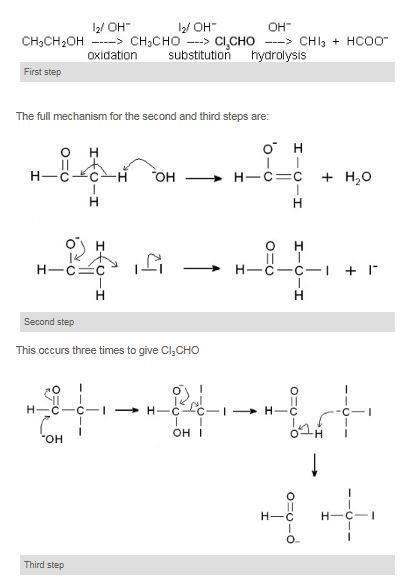
A Test To Distinguish Between Ethanol And Methanol Experiment Rsc Education

No comments for "Iodoform Test for Alcohols"
Post a Comment